No products in the cart.
GHK Basic 200mg (Tripeptide-1) (Topical)
$170.00
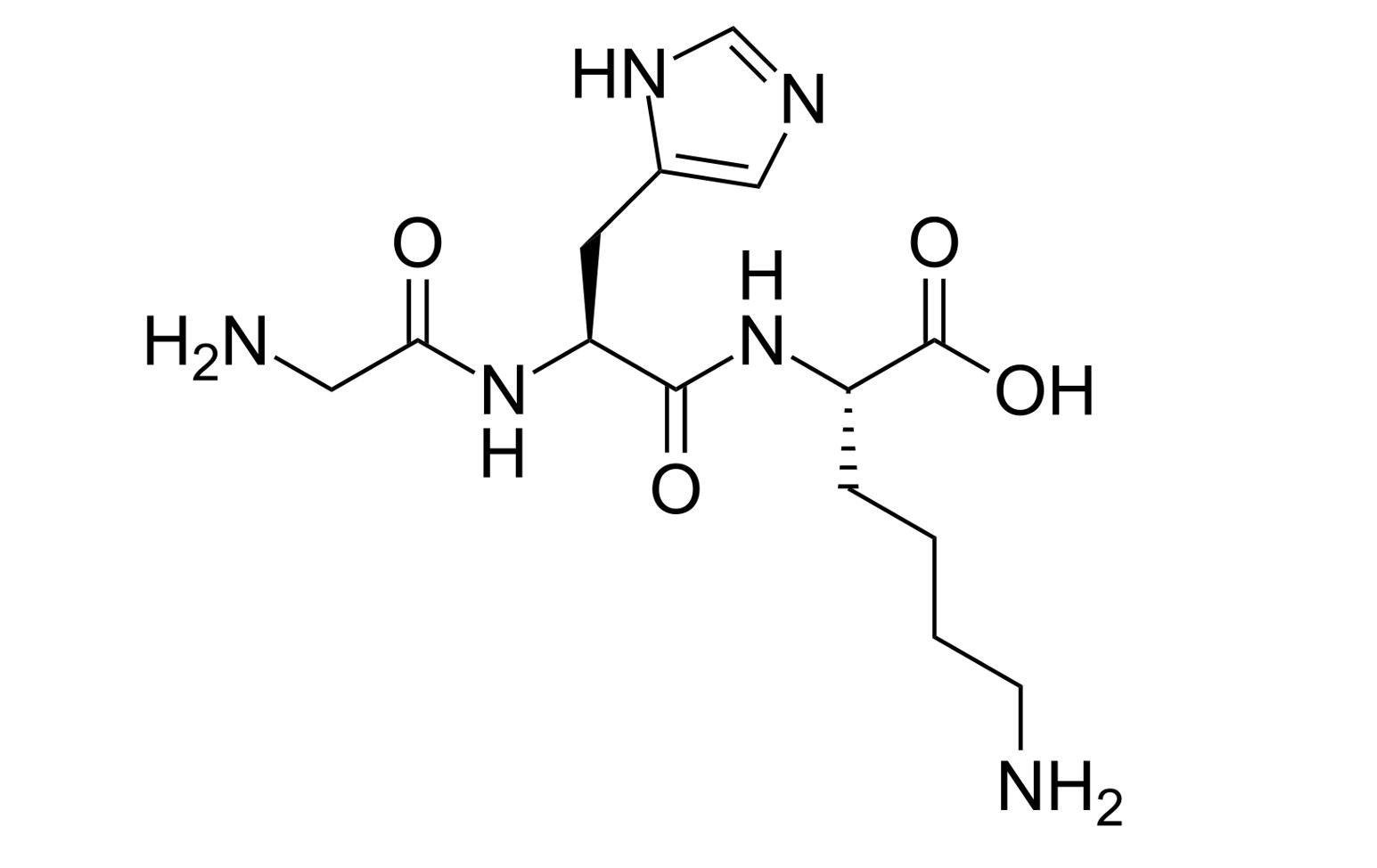
GHK Basic (Tripeptide-1) is a research peptide consisting of the sequence Gly-His-Lys, a naturally occurring fragment studied for its role in extracellular matrix regulation and antioxidant defense. It influences fibroblast activation, collagen gene expression, and metalloprotein regulation under controlled laboratory conditions. GHK Basic is used as a model for studying peptide-mediated cellular regeneration and redox homeostasis.
Category: Cosmetic Peptides
GHK is a tripeptide with the amino acid sequence glycyl-histidyl-lysine. In preclinical research, GHK is used as a tool compound to evaluate peptide-driven modulation of cellular stress-response programs, extracellular matrix (ECM)-associated transcriptional networks, and metal-ion (Cu) complexation effects. GHK is frequently studied both as the free tripeptide and as a copper complex (GHK-Cu) to compare how transition-metal coordination influences physicochemical behavior and downstream signaling readouts in cellular and in vivo model systems.
Biochemical Characteristics
Identity: GHK (Glycyl-Histidyl-Lysine), tripeptide
Complexation: Commonly evaluated as apo-GHK and as a Cu(II) coordination complex (GHK-Cu) in mechanistic studies
The histidine and terminal amine functionalities in GHK can coordinate metal ions, and copper complexation is used experimentally to probe how redox-active cofactors and peptide coordination chemistry impact protein-binding interactions, gene-expression signatures, and cellular oxidative-stress handling under controlled laboratory conditions.
Research Applications
- Transcriptomic profiling: Assessment of GHK-associated gene-expression changes across stress, proteostasis, inflammatory, and ECM-related pathways in cultured cells and preclinical models.
- Peptide–metal coordination studies: Comparative evaluation of apo-GHK vs GHK-Cu to examine how Cu(II) coordination alters peptide stability, binding behavior, and redox-associated pathway readouts.
- Proteostasis / UPS research: Use in studies examining ubiquitin–proteasome system (UPS) gene programs and protein quality-control signaling.
- ECM & remodeling models: Investigation of ECM transcriptional markers, matrix-associated proteins, and remodeling-associated signaling networks (including TGF superfamily-linked gene programs) in cell and animal models.
- Inflammation-related readouts: Evaluation of cytokine-linked mechanisms (e.g., IL-6-associated programs) and downstream acute-phase gene networks in relevant cellular systems.
- Insulin/IGF-like signaling exploration: Use as a mechanistic probe for insulin/IGF-like pathway gene sets in preclinical gene-expression datasets.
Pathway / Mechanistic Context
GHK vs GHK-Cu (conceptual distinction): In experimental designs, GHK is evaluated either as the free tripeptide or as a copper coordination complex (GHK-Cu). Copper coordination can influence peptide conformation, charge distribution, and redox behavior, which may shift pathway readouts in assays focused on oxidative-stress handling, transcriptional regulation, and matrix-associated signaling.
Copper as a redox-active cofactor: Copper is a transition metal used by numerous enzymes that rely on Cu(I)/Cu(II) interconversion to support electron-transfer chemistry. In biological systems, copper-dependent enzymes are involved in processes such as cellular respiration, antioxidant defense, and connective-tissue crosslinking. In RUO contexts, copper–peptide coordination is used to study how metal availability and ligand binding can affect pathway-level signaling and gene expression under controlled conditions.
ECM-linked peptide fragments: Short peptide motifs associated with matrix proteins (including sequences present in collagen-associated proteins such as SPARC) are frequently used as research tools to examine remodeling-associated programs and cell–matrix signaling. GHK is discussed in the literature as a motif relevant to matrix-associated turnover and remodeling models.
Preclinical Research Summary
1. Fibrinogen / IL-6-Linked Gene Programs
Preclinical gene-expression analyses summarized in the referenced literature describe GHK-associated modulation of inflammatory and acute-phase signaling, including IL-6-linked programs and fibrinogen-chain gene sets (e.g., FGB). These observations are typically reported from in vitro cellular systems and transcriptomic datasets used to infer pathway-level effects.
2. Ubiquitin/Proteasome System (UPS)
Reported transcriptional datasets include enrichment of ubiquitin–proteasome system (UPS) gene programs, a pathway class central to protein quality control and removal of damaged proteins. RUO studies use these readouts to map proteostasis-linked responses in cultured cells under defined experimental perturbations.
3. DNA Repair Gene Sets
Published analyses describe associations between GHK exposure and altered expression of DNA repair-related genes in in vitro models. In an RUO setting, these data are used as mechanistic background for selecting endpoints and validating pathway enrichment rather than for any intended-use interpretation.
4. Oxidative-Stress / Antioxidant Gene Programs
Gene-expression summaries in the cited references report changes in antioxidant and oxidative-stress-associated gene panels following GHK or GHK-Cu exposure in experimental systems. Such datasets are frequently used to study redox-response signaling and copper-dependent effects in cell culture.
5. Insulin / IGF-Like Signaling Signatures
Transcriptomic analyses discussed in the cited literature include modulation of insulin/IGF-like pathway gene sets. In preclinical research, these signatures are typically evaluated as part of broader systems-biology analyses to understand pathway coupling between metabolism-linked networks and stress-response programs.
6. TGF Superfamily-Associated Remodeling Programs
The referenced publications discuss GHK-associated gene programs that intersect with TGF superfamily signaling, a pathway group that regulates cell differentiation, ECM remodeling, and tissue-architecture-related transcriptional responses in many model systems. RUO studies may use these observations to guide mechanistic hypotheses and downstream validation experiments.
7. Cancer-Related Gene-Set Reversal Analyses
The cited literature includes discussions of gene-expression signature analyses in which compounds (including GHK in specified in vitro conditions) are evaluated for their ability to reverse predefined disease-associated transcriptional patterns. In an RUO context, these approaches are used as computational and experimental tools for pathway mapping and hypothesis generation in oncology-related model systems, without implying any intended use.
Conclusion
Across the cited publications, GHK and GHK-Cu are described primarily as research tools for probing multi-pathway gene expression, including ECM remodeling, proteostasis (UPS), oxidative-stress responses, and growth-factor/cytokine-linked signaling. These observations derive from preclinical datasets (cell culture systems, transcriptomic analyses, and model-based investigations) and are used to inform experimental design, endpoint selection, and mechanistic interpretation [1–4].
Any pharmacokinetic, tolerability, or administration statements from nonclinical reports should be interpreted strictly as preclinical background for experimental planning and are not indicative of suitability for any intended-use context.
Be the first to review “GHK Basic 200mg (Tripeptide-1) (Topical)” Cancel reply
Related products
Cosmetic Peptides
$199.00
Cosmetic Peptides
$85.00
Cosmetic Peptides
$180.00
Cosmetic Peptides
$189.00
Cosmetic Peptides
$199.00
Cosmetic Peptides
$200.00 – $499.00









Reviews
There are no reviews yet.